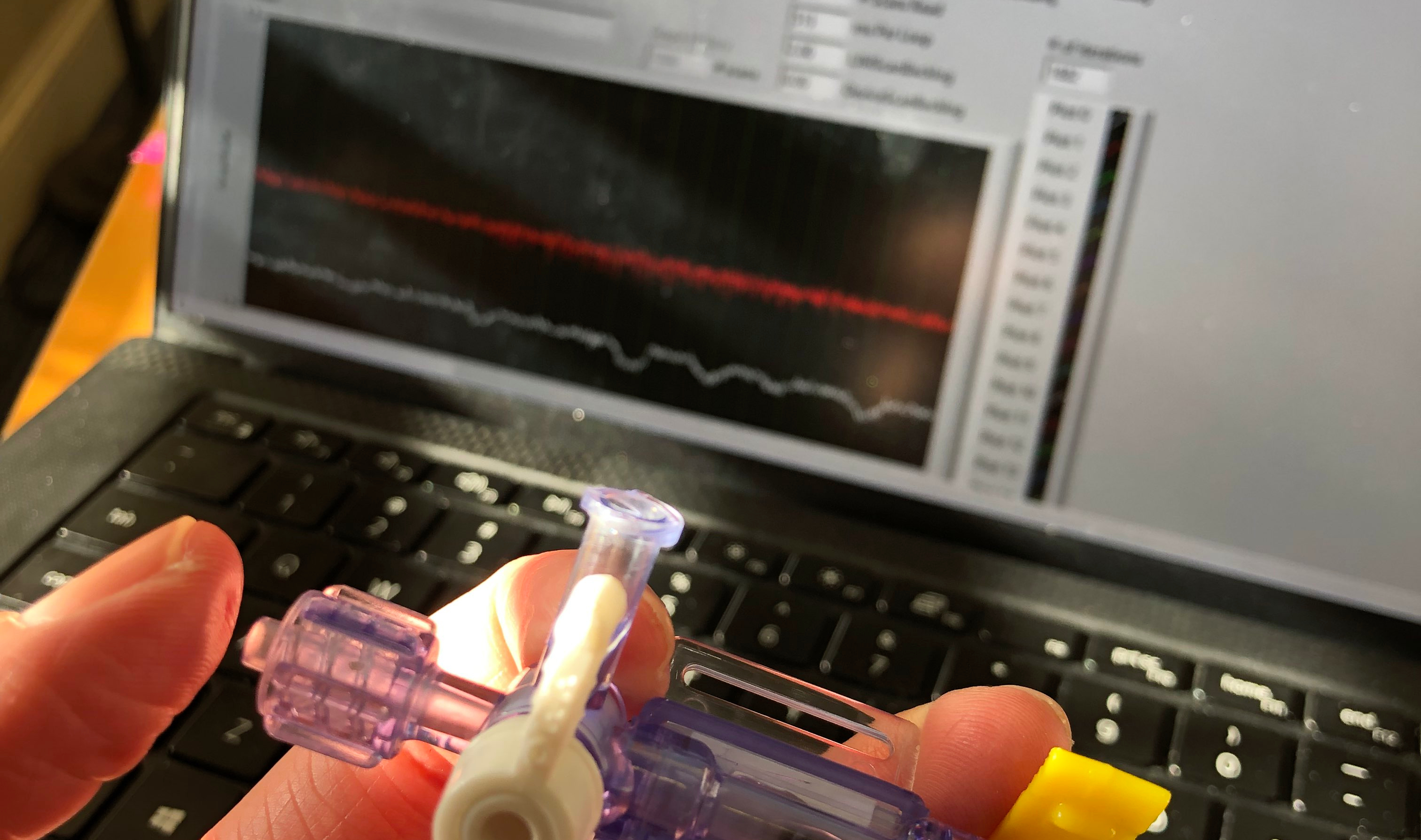
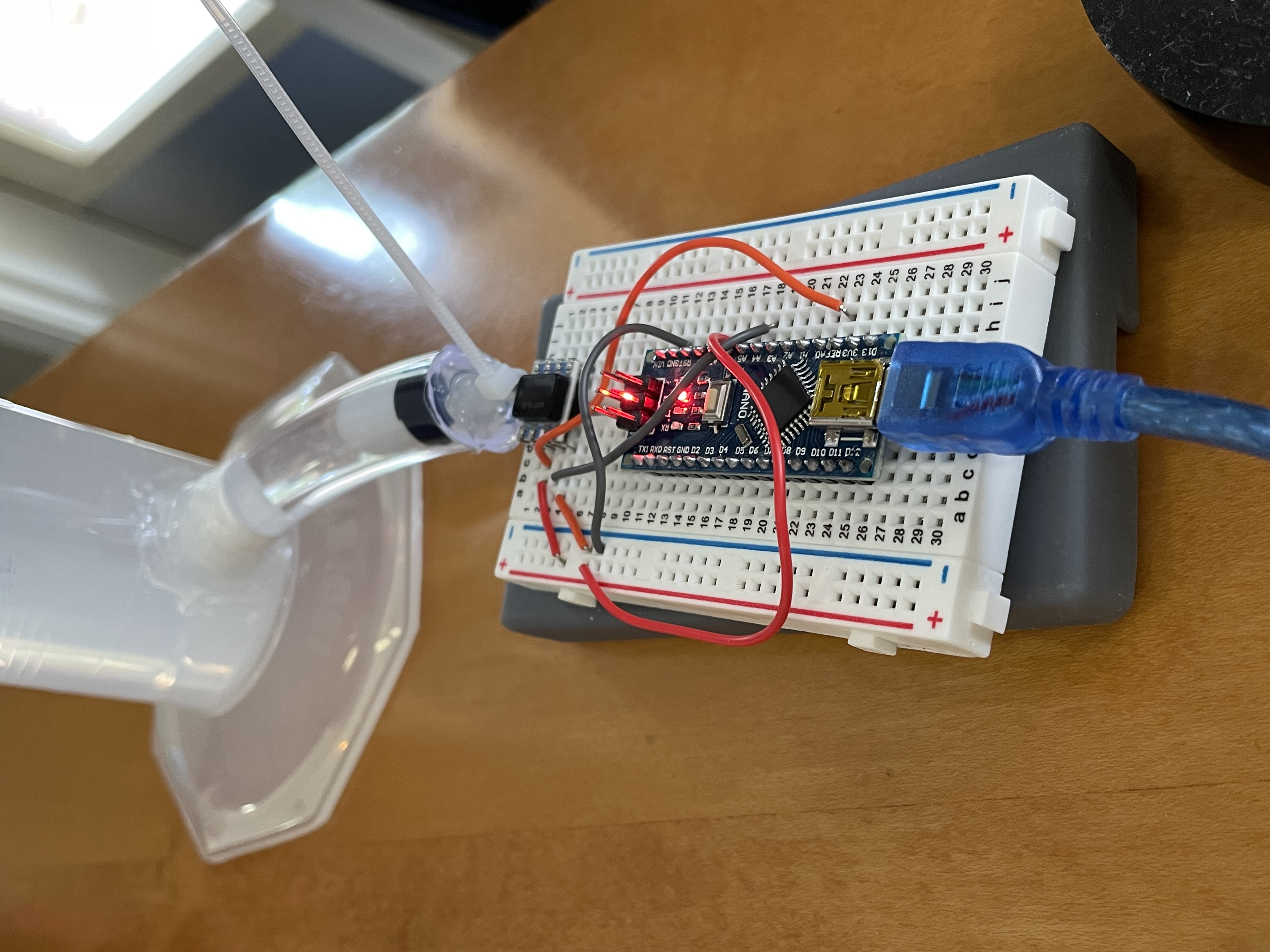
Insterstitial Fluid Pressure Sensor
Our device continuously measures changes in the interstitial fluid pressure for early detection of heart failure through a minimally invasive procedure involving a portable needle-based application.
- Minimally invasive
- Real-time tracking
- Portable
Better monitoring your cardiovascular health.
Minimally invasive
No surgery needed! Our insertion system is easy to install and less cumbersome. Device application and removal can be done at home.
Real-time tracking
The sensor continuously collects data and visualizes in real-time manner to your smartphone.
Portable
We designed it for your convenience! Our device strongly adheres to your skin, even when you are running, exercising or playing sports.
Insterstitial Fluid Pressure Sensor

A way to address a continuous and minimally invasive measuring interstitial fluid pressure in cardiovascular diseases patients that help monitor heart health and heart failure.
market
Customers who should consider this product should be ages 20 and older in the U.S. who exhibit symptoms of heart failure. Individuals who experience symptoms of heart failure could be caused by stress and/or have an unhealthy diet. About 18.2 million adults, ages 20 and older suffer from Coronary Heart Disease which leads to heart failure. The Interstitial Fluid Pressure Sensor will allow patients and their physician to monitor the persisting symptoms and take actions to reduce worsening symptoms. Our product will be the first product to be a minimally invasive, continuous, and portable heart failure diagnostic tool. The device will be offered at hospitals and pharmacies. In order for an individual to obtain this device, they must seek a prescription from their physician.
Competitor
There are currently no devices on the market which measure interstitial fluid pressure (IFP) directly as an indicator for heart failure. Clinically, to diagnose IFP, the reliable devices all involve invasive techniques. For example, the leading device by Abbott, CardioMEMS, is able to determine heart failure worsening reliably through the pulmonary artery, however, the device requires implantation. The current market for cardiovascular diseases is around $54.08 billion where heart failure is a major part. The market share of even a small portion will be highly impactful.
We have partnerships with



Team
Meet our team

Ethan Hoang
Product Manager"A product manager not only requires knowledge of product but knowledge of people."

Tam Vo
Quality Engineer"Ensure the highest quality and safety level of each product."

Tyler Hanwinyoo
Manufacturing Engineer"There's always a more optimal production process."

Emily Quach
Product Engineer"The best way to predict the future is to create it."